Mounting evidence suggests that gut commensal bacteria play an important role in shaping immune responses in mice and humans.
We are investigating whether people with MS harbor a different kind of gut bacteria than healthy individuals.
Our work involves identifying and characterizing gut bacterial communities by sequencing DNA from stool in large populations patients, testing the effect of specific bacteria on immune cells and evaluating the consequence of transplanting human microbiota into germ-free mice. Some of our projects include:
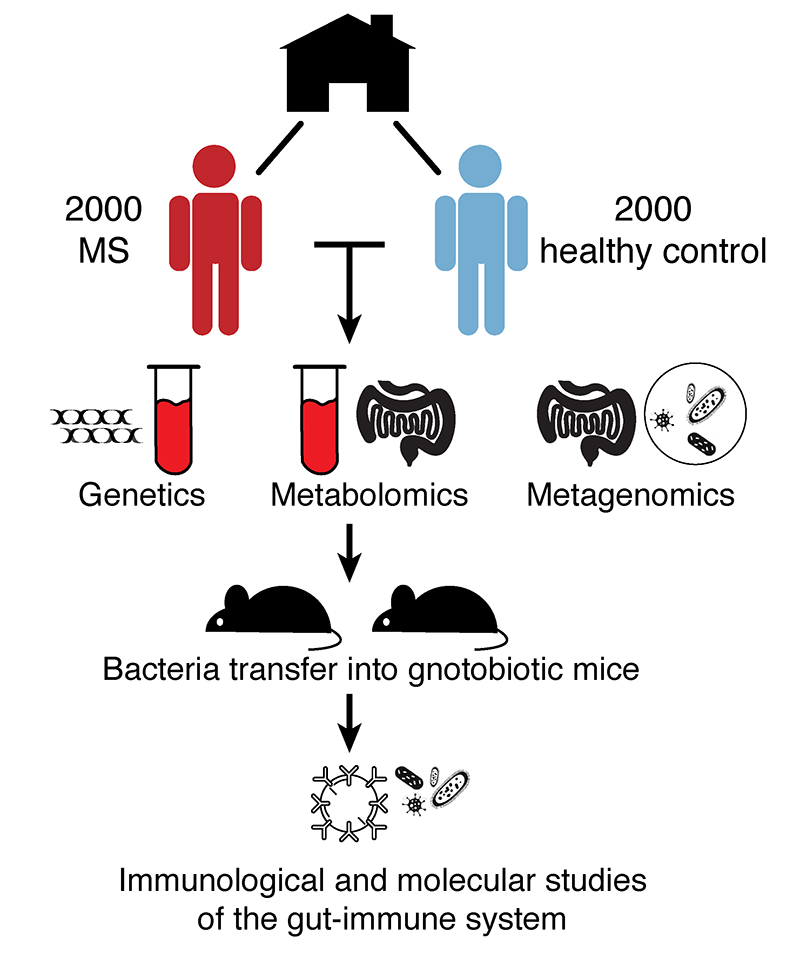
International MS Microbiome Study (iMSMS)
We lead the international MS microbiome study, the largest global effort to map the gut microbiota in MS. The iMSMS uses a meticulous study design, in which cases and controls are from the same household (typically spouses or housemates). This design guarantees minimizing large sources of variance in the microbiome such as diet, geographical location and age. The iMSMS currently recruits patients in the United States, Europe and South America.
Cross-talk Between Gut Bacteria and Immune Cells
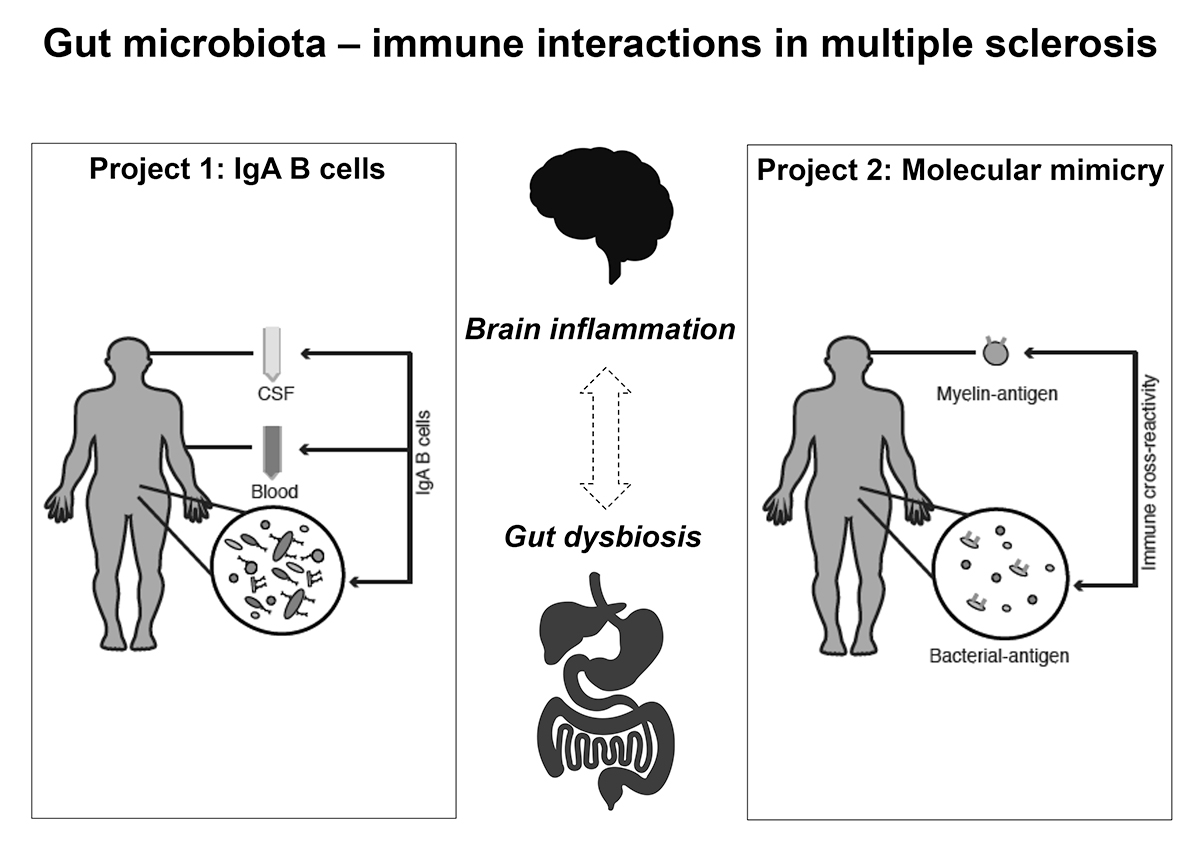
We hypothesize that bacteria more prevalent in MS will have apro-inflammatory role and bacteria more prevalent in healthy controls will have an immune-regulatory role. We are also interested in the role of IgA-producing cells in regulating gut microbiota and their potential influence in brain inflammation. Another lead we are following relates to the potential molecular mimicry between bacterial and CNS antigens
Clinical Trial with Fecal Microbiota Transplantation (FMT)
We are conducting MS-Biome, the first phase-1 clinical trial using FMT in relapsing MS patients. Primary endpoints are safety, tolerability and donor microbiome engraftment. Secondary endpoints include evaluation of whether FMT of donor stool can induce a shift from pro-inflammatory to immunomodulatory T cell profiles, changes in humoral function and influence clinical and radiological endpoints.
Clinical Coordinator Team
Crystal Luong (iMSMS Study Coordinator)
Crystal runs most of the Baranzini Lab’s clinical operations, which include recruiting and consenting patients for the iMSMS and the microbiome components of EPIC and ORIGINS studies; managing data collection; and working with the lab to ensure proper sample acquisition. Crystal works with MS centers around the world to bring them on board as collaborating sites within the International Multiple Sclerosis Microbiome Study (iMSMS). Sam is the coordinator of the MS-Biome study.
Germ-free Mouse Facility
Mehrzad Modabber (Manager)
The Sandler Gnotobiotic Animal Facility (SGAF) is equipped with a state-of the-art Tecniplast IsoCage P—Bioexclusion system. This system is specifically designed for studies using germ-free, gnotobiotic or immunocompromised mice. Each cage is its own isolator, is airtight and contains a HEPA filter with high positive pressure for ideal bioexclusion. The facility has been in service since 2017. During this time, we have successfully bred and maintained C57BL/6 and 2D2 transgenic mouse lines. We gradually continue to add and derive new strains GF to supply incoming research projects.
